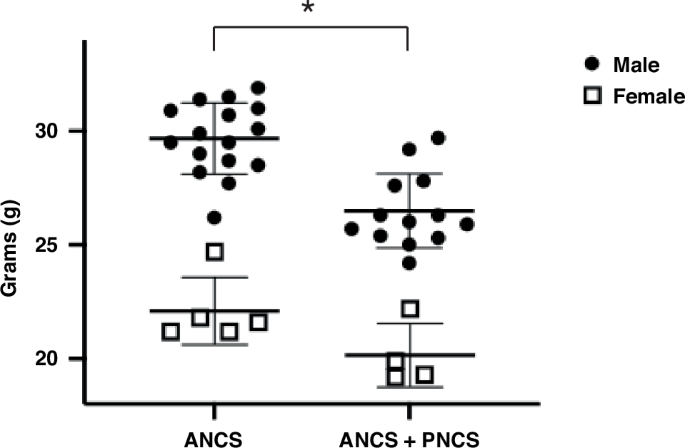
Long-term multi-organ system abnormalities in mice exposed to antenatal and postnatal corticosteroids
Crump, C. An overview of adult health outcomes after preterm birth. Early Hum. Dev. 150, 105187, (2020).
Google Scholar
Lurbe, E. & Ingelfinger, J. Developmental and early life origins of cardiometabolic risk factors: novel findings and implications. Hypertension 77, 308–318, (2021).
Google Scholar
Rog-Zielinska, E. A., Richardson, R. V., Denvir, M. A. & Chapman, K. E. Glucocorticoids and foetal heart maturation; implications for prematurity and foetal programming. J. Mol. Endocrinol. 52, R125–R135, (2014).
Google Scholar
Asztalos, E. Antenatal corticosteroids: a risk factor for the development of chronic disease. J. Nutr. Metab. 2012, 930591, (2012).
Google Scholar
Busada, J. T. & Cidlowski, J. A. Mechanisms of glucocorticoid action during development. Curr. Top. Dev. Biol. 125, 147–170, (2017).
Google Scholar
Braun, T., Challis, J. R., Newnham, J. P. & Sloboda, D. M. Early-life glucocorticoid exposure: the hypothalamic-pituitary-adrenal axis, placental function, and long-term disease risk. Endocr. Rev. 34, 885–916, (2013).
Google Scholar
Committee on Obstetric, P Committee opinion No. 713: antenatal corticosteroid therapy for fetal maturation. Obstet. Gynecol. 130, e102–e109, (2017).
Google Scholar
Kemp, M. W. et al. Efficacy and safety of antenatal steroids. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315, R825–R839, (2018).
Google Scholar
Roberts, D., Brown, J., Medley, N. & Dalziel, S. R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 3, CD004454, (2017).
Google Scholar
Doyle, L. W., Cheong, J. L., Hay, S., Manley, B. J. & Halliday, H. L. Late (>/= 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 11, CD001145, (2021).
Google Scholar
Lok, I. M. et al. Effects of postnatal corticosteroids on lung development in newborn animals. A systematic review. Pediatr. Res. (2024).
Harris, C. et al. Postnatal dexamethasone exposure and lung function in adolescents born very prematurely. PLoS One 15, e0237080, (2020).
Google Scholar
Vrselja, A., Pillow, J. J. & Black, M. J. Effect of preterm birth on cardiac and cardiomyocyte growth and the consequences of antenatal and postnatal glucocorticoid treatment. J. Clin. Med. 10 (2021).
Festing, M. F. & Altman, D. G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 43, 244–258, (2002).
Google Scholar
Li, H., Yuan, X., Tang, J. & Zhang, Y. Lipopolysaccharide disrupts the directional persistence of alveolar myofibroblast migration through EGF receptor. Am. J. Physiol. Lung Cell Mol. Physiol. 302, L569–L579, (2012).
Google Scholar
Cooney, T. P. & Thurlbeck, W. M. The radial alveolar count method of Emery and Mithal: a reappraisal 1–postnatal lung growth. Thorax 37, 572–579, (1982).
Google Scholar
Emery, J. L. & Mithal, A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch. Dis. Child 35, 544–547, (1960).
Google Scholar
Xu, J. et al. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ. Res. 98, 342–350, (2006).
Google Scholar
Wallner, M. et al. Acute catecholamine exposure causes reversible myocyte injury without cardiac regeneration. Circ. Res 119, 865–879, (2016).
Google Scholar
Hillman, N. H. et al. Dose of budesonide with surfactant affects lung and systemic inflammation after normal and injurious ventilation in preterm lambs. Pediatr. Res. 88, 726–732, (2020).
Google Scholar
Cummings, J. J., Pramanik, A. K., Committee On, F. & Newborn. Postnatal corticosteroids to prevent or treat chronic lung disease following preterm birth. Pediatrics 149 (2022).
Jobe, A. H., Milad, M. A., Peppard, T. & Jusko, W. J. Pharmacokinetics and pharmacodynamics of intramuscular and oral betamethasone and dexamethasone in reproductive age women in India. Clin. Transl. Sci. 13, 391–399, (2020).
Google Scholar
Tijsseling, D. et al. Neonatal corticosteroid therapy affects growth patterns in early infancy. PLoS One 13, e0192162, (2018).
Google Scholar
Regan, F. M., Cutfield, W. S., Jefferies, C., Robinson, E. & Hofman, P. L. The impact of early nutrition in premature infants on later childhood insulin sensitivity and growth. Pediatrics 118, 1943–1949, (2006).
Google Scholar
Singhal, A. Early nutrition and long-term cardiovascular health. Nutr. Rev. 64, S44–S49, (2006). discussion S72-91.
Google Scholar
Singhal, A., Cole, T. J., Fewtrell, M., Deanfield, J. & Lucas, A. Is slower early growth beneficial for long-term cardiovascular health? Circulation 109, 1108–1113, (2004).
Google Scholar
Qin, G. et al. Postnatal dexamethasone, respiratory and neurodevelopmental outcomes at two years in babies born extremely preterm. PLoS One 12, e0181176, (2017).
Google Scholar
Harris, C. et al. Effect of dexamethasone exposure on the neonatal unit on the school age lung function of children born very prematurely. PLoS One 13, e0200243, (2018).
Google Scholar
Evans, N. Cardiovascular effects of dexamethasone in the preterm infant. Arch. Dis. Child Fetal Neonatal Ed. 70, F25–F30, (1994).
Google Scholar
Gill, A. W., Warner, G. & Bull, L. Iatrogenic neonatal hypertrophic cardiomyopathy. Pediatr. Cardiol. 17, 335–339, (1996).
Google Scholar
Werner, J. C. et al. Hypertrophic cardiomyopathy associated with dexamethasone therapy for bronchopulmonary dysplasia. J. Pediatr. 120, 286–291, (1992).
Google Scholar
Zecca, E. et al. Cardiac adverse effects of early dexamethasone treatment in preterm infants: a randomized clinical trial. J. Clin. Pharm. 41, 1075–1081, (2001).
Google Scholar
Skelton, R., Gill, A. B. & Parsons, J. M. Cardiac effects of short course dexamethasone in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 78, F133–F137, (1998).
Google Scholar
Ohning, B. L., Fyfe, D. A. & Riedel, P. A. Reversible obstructive hypertrophic cardiomyopathy after dexamethasone therapy for bronchopulmonary dysplasia. Am. Heart J. 125, 253–256, (1993).
Google Scholar
de Vries, W. B. et al. Alterations in adult rat heart after neonatal dexamethasone therapy. Pediatr. Res 52, 900–906, (2002).
Google Scholar
Bal, M. P. et al. Histopathological changes of the heart after neonatal dexamethasone treatment: studies in 4-, 8-, and 50-week-old rats. Pediatr. Res 66, 74–79, (2009).
Google Scholar
Niu, Y., Herrera, E. A., Evans, R. D. & Giussani, D. A. Antioxidant treatment improves neonatal survival and prevents impaired cardiac function at adulthood following neonatal glucocorticoid therapy. J. Physiol. 591, 5083–5093, (2013).
Google Scholar
Bal, M. P. et al. Long-term cardiovascular effects of neonatal dexamethasone treatment: hemodynamic follow-up by left ventricular pressure-volume loops in rats. J. Appl Physiol. (1985) 104, 446–450, (2008).
Google Scholar
Kamphuis, P. J. et al. Reduced life expectancy in rats after neonatal dexamethasone treatment. Pediatr. Res 61, 72–76, (2007).
Google Scholar
Jiang, X. et al. Effects of neonatal dexamethasone administration on cardiac recovery ability under ischemia-reperfusion in 24-wk-old rats. Pediatr. Res. 80, 128–135, (2016).
Google Scholar
Cardoso, R. C. & Padmanabhan, V. Prenatal steroids and metabolic dysfunction: lessons from sheep. Annu. Rev. Anim. Biosci. 7, 337–360, (2019).
Google Scholar
Fowden, A. L., Giussani, D. A. & Forhead, A. J. Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 21, 29–37, (2006).
Google Scholar
Jellyman, J. K., Valenzuela, O. A. & Fowden, A. L. HORSE SPECIES SYMPOSIUM: glucocorticoid programming of hypothalamic-pituitary-adrenal axis and metabolic function: Animal studies from mouse to horse. J. Anim. Sci. 93, 3245–3260, (2015).
Google Scholar
Leone, T. C. et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 3, e101, (2005).
Google Scholar
Besse-Patin, A. et al. Estrogen signals through peroxisome proliferator-activated receptor-gamma coactivator 1alpha to reduce oxidative damage associated with diet-induced fatty liver disease. Gastroenterology 152, 243–256, (2017).
Google Scholar
Estall, J. L. et al. Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic peroxisome proliferator-activated receptor-gamma coactivator-1alpha expression. Diabetes 58, 1499–1508, (2009).
Google Scholar
Kleiner, S. et al. Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proc. Natl. Acad. Sci. USA 109, 9635–9640, (2012).
Google Scholar
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217, (2013).
Google Scholar
Zhang, Y. et al. Mitochondrial aldehyde dehydrogenase 2 accentuates aging-induced cardiac remodeling and contractile dysfunction: role of AMPK, Sirt1, and mitochondrial function. Free Radic. Biol. Med. 71, 208–220, (2014).
Google Scholar
Lemieux, H., Vazquez, E. J., Fujioka, H. & Hoppel, C. L. Decrease in mitochondrial function in rat cardiac permeabilized fibers correlates with the aging phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 65, 1157–1164, (2010).
Google Scholar
Owesny, P. & Grune, T. The link between obesity and aging – insights into cardiac energy metabolism. Mech. Ageing Dev. 216, 111870. (2023).
Google Scholar
Balsan, G. A., Vieira, J. L., Oliveira, A. M. & Portal, V. L. Relationship between adiponectin, obesity and insulin resistance. Rev. Assoc. Med. Bras. (1992) 61, 72–80, (2015).
Google Scholar
Jung, U. J. & Choi, M. S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 15, 6184–6223, (2014).
Google Scholar
Hulthe, J., Hulten, L. M. & Fagerberg, B. Low adipocyte-derived plasma protein adiponectin concentrations are associated with the metabolic syndrome and small dense low-density lipoprotein particles: atherosclerosis and insulin resistance study. Metabolism 52, 1612–1614, (2003).
Google Scholar
Crume, T. L. et al. The long-term impact of intrauterine growth restriction in a diverse U.S. cohort of children: the EPOCH study. Obesity (Silver Spring) 22, 608–615, (2014).
Google Scholar
Ordonez-Diaz, M. D. et al. Plasma adipokines profile in prepubertal children with a history of prematurity or extrauterine growth restriction. Nutrients 12 (2020).
Dai, Y. et al. Prenatal prednisone exposure impacts liver development and function in fetal mice and its characteristics. Toxicol. Sci. 199, 63–80, (2024).
Google Scholar
Nair, A. B. & Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 7, 27–31, (2016).
Google Scholar
Schittny, J. C. Development of the lung. Cell Tissue Res. 367, 427–444, (2017).
Google Scholar
Nguyen, T. & Jordan, B. K. Let’s talk about dex: when do the benefits of dexamethasone for prevention of bronchopulmonary dysplasia outweigh the risks?. Newborn (Clarksville) 1, 91–96, (2022).
Google Scholar
Dutta, S. & Sengupta, P. Men and mice: relating their ages. Life Sci. 152, 244–248, (2016).
Google Scholar
Effect of corticosteroids for fetal maturation on perinatal outcomes NIH consensus development panel on the effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA 273, 413–418, (1995).
Google Scholar
Kim, Y. E., Park, W. S., Sung, D. K., Ahn, S. Y. & Chang, Y. S. Antenatal betamethasone enhanced the detrimental effects of postnatal dexamethasone on hyperoxic lung and brain injuries in newborn rats. PLoS One 14, e0221847, (2019).
Google Scholar
Ersek, A. et al. Strain dependent differences in glucocorticoid-induced bone loss between C57BL/6J and CD-1 mice. Sci. Rep. 6, 36513. (2016).
Google Scholar
Hodes, G. E. et al. Strain differences in the effects of chronic corticosterone exposure in the hippocampus. Neuroscience 222, 269–280, (2012).
Google Scholar
Shelton, E. L. et al. Effects of antenatal betamethasone on preterm human and mouse ductus arteriosus: comparison with baboon data. Pediatr. Res. 84, 458–465, (2018).
Google Scholar
Zhang, H. et al. The angiogenic factor midkine is regulated by dexamethasone and retinoic acid during alveolarization and in alveolar epithelial cells. Respir. Res. 10, 77, (2009).
Google Scholar
Krishnan, A. et al. A detailed comparison of mouse and human cardiac development. Pediatr. Res. 76, 500–507, (2014).
Google Scholar
Wessels, A. & Sedmera, D. Developmental anatomy of the heart: a tale of mice and man. Physiol. Genom. 15, 165–176, (2003).
Google Scholar
Kaffe, E. et al. Humanized mouse liver reveals endothelial control of essential hepatic metabolic functions. Cell 186, 3793–3809 e3726, (2023).
Google Scholar
Vickers, M. H. Developmental programming and transgenerational transmission of obesity. Ann. Nutr. Metab. 64, 26–34, (2014).
Google Scholar
Buescher, J. L. et al. Evidence for transgenerational metabolic programming in Drosophila. Dis. Model Mech. 6, 1123–1132, (2013).
Google Scholar
Zhu, Z., Cao, F. & Li, X. Epigenetic programming and fetal metabolic programming. Front Endocrinol. (Lausanne) 10, 764, (2019).
Google Scholar
link